Water Nitrate Remote Monitoring System with Self-Diagnostic Function for Ion-Selective Electrodes
The detection of nitrate pollutants is a widely used strategy for protecting water sources. Although ion-selective electrodes (ISEs).
1. Introduction
2. Materials and Methods
2.1. Configuration of IoT-Based Nitrate Measurement System
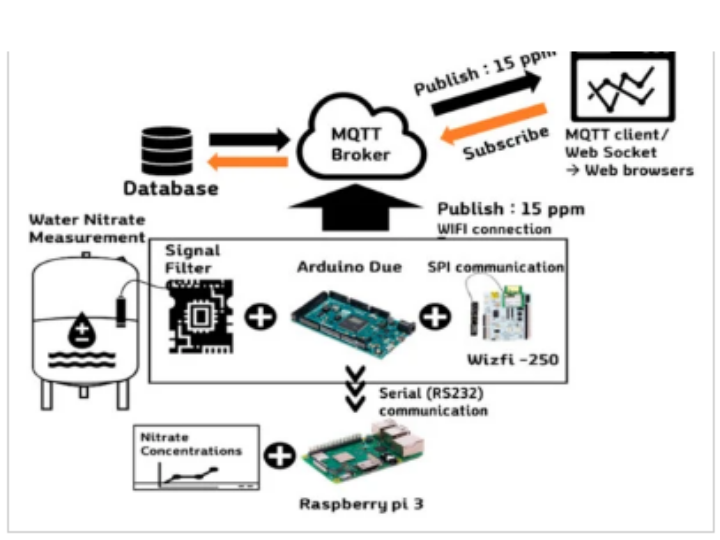
The developed system communicates with the cloud server by using the message queuing telemetry transport (MQTT) protocol and provides monitoring information through the developed web-based monitoring page. A web-based nitrate monitoring page was constructed and tested to monitor measurement data on the web. As shown in Figure 1, the message queuing telemetry transport (MQTT) protocol was implemented through the Arduino Due-embedded board, thereby enabling it to recognize the broker server via the wireless network and to publish data from the three nitrate electrodes [26]. Moreover, the web-based nitrate monitoring page was constructed and tested to monitor measurement data on the web. The data are published on the broker’s own webpage and sent on to the user. The web page was hosted using a commercial cloud server (Naver Cloud Platform, Naver, Seongnam, Korea) and the server configuration was Node.JS, and Vue.JS was used for building a front-end UI.
The overall sequence of the developed water nitrate monitoring system with self-diagnostic function is shown in Figure 4. The sequence includes a two-point normalization setup with rinsing, sampling pumps, drainage via a solenoid valve, and measurements with the ISEs, while the data communication processes with the MQTT server and fault diagnosis are presented. The two-point normalization method consisting of a sensitivity adjustment followed by an offset adjustment was used to minimize the potential drift. Two different concentrations of 10 and 100 mg L−1 were used as known standard solutions of low and high concentrations, respectively, to determine the slope and offset values prior to sample measurement. Through this process, the signal drift, sensitivity, and deviation values were obtained for each electrode required for the self-test function.
2.2. Self-Diagnostic Algorithm for ISE
Selection of SDI for Electrode
The change in sensitivity is an essential factor for assessing the performance of the ISE [14]. In addition, the continuous data from sensor signals must be analyzed to determine whether there is a signal drift (which is also an important index for assessing the sensor performance). In this study, the current status of the nitrate electrodes was quantitatively checked using three indicators, i.e., electrode drift index (S1), sensitivity change index (S2), and estimated value change index between multiple electrodes (S3):
The SDI (%) is presented in Equation (4):
2.2. Self-Diagnostic Algorithm for ISE
Selection of SDI for Electrode
The change in sensitivity is an essential factor for assessing the performance of the ISE [14]. In addition, the continuous data from sensor signals must be analyzed to determine whether there is a signal drift (which is also an important index for assessing the sensor performance). In this study, the current status of the nitrate electrodes was quantitatively checked using three indicators, i.e., electrode drift index (S1), sensitivity change index (S2), and estimated value change index between multiple electrodes.